

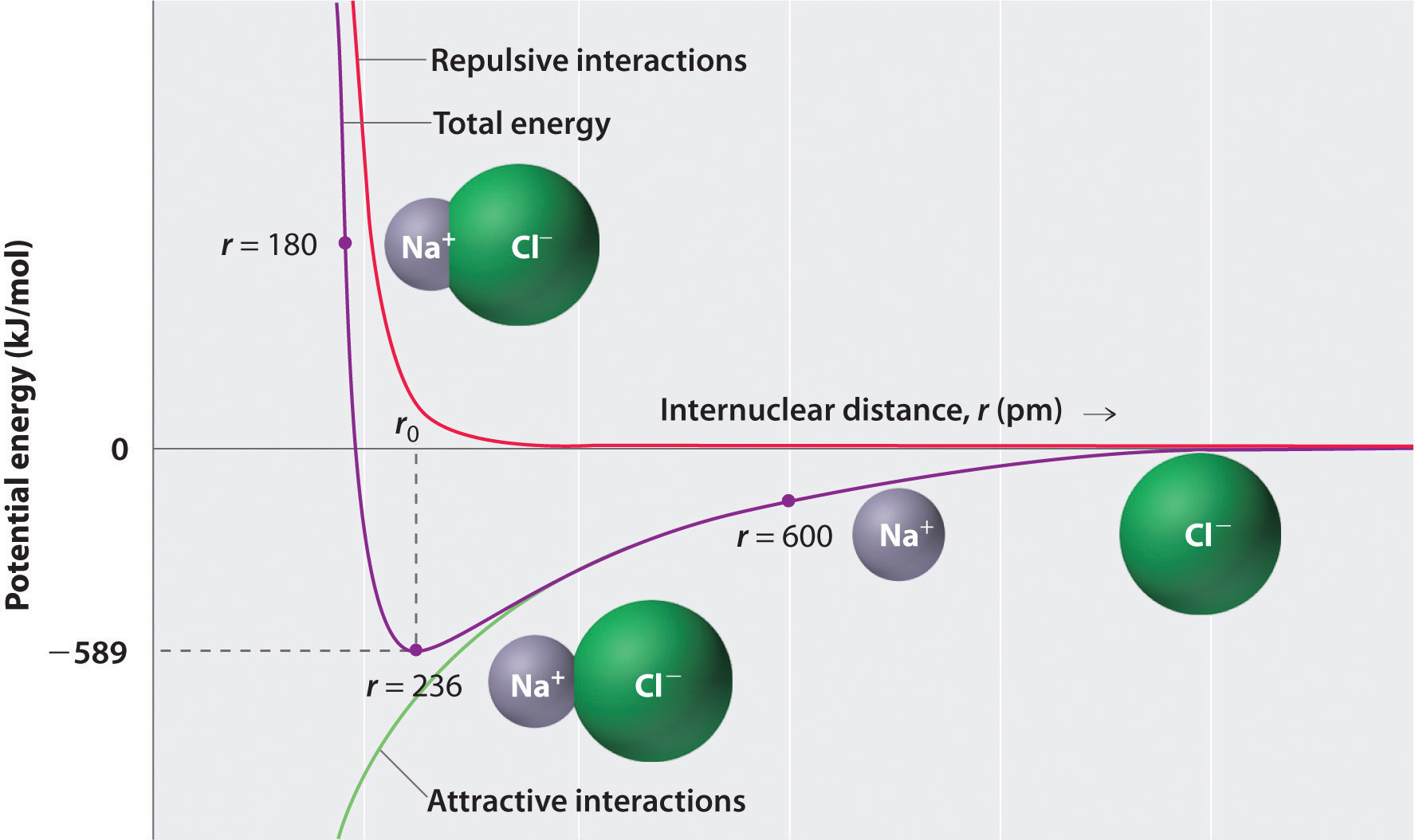
When sodium gives up that electron, it now has 11 positive charges in its nucleus, but only 10 electrons, so it’s a +1 ion. And something else happens in the process. I don’t want it.” And so, the sodium’s happy it has 10 chlorine’s happy, it has 18. Well, naturally the sodium says, “Here, take my electron. So, imagine what happens when a chlorine atom meets a sodium atom. By the same token, sodium, which is element 11, has one too many electrons, and it’s going to do almost anything it can to lose an electron.
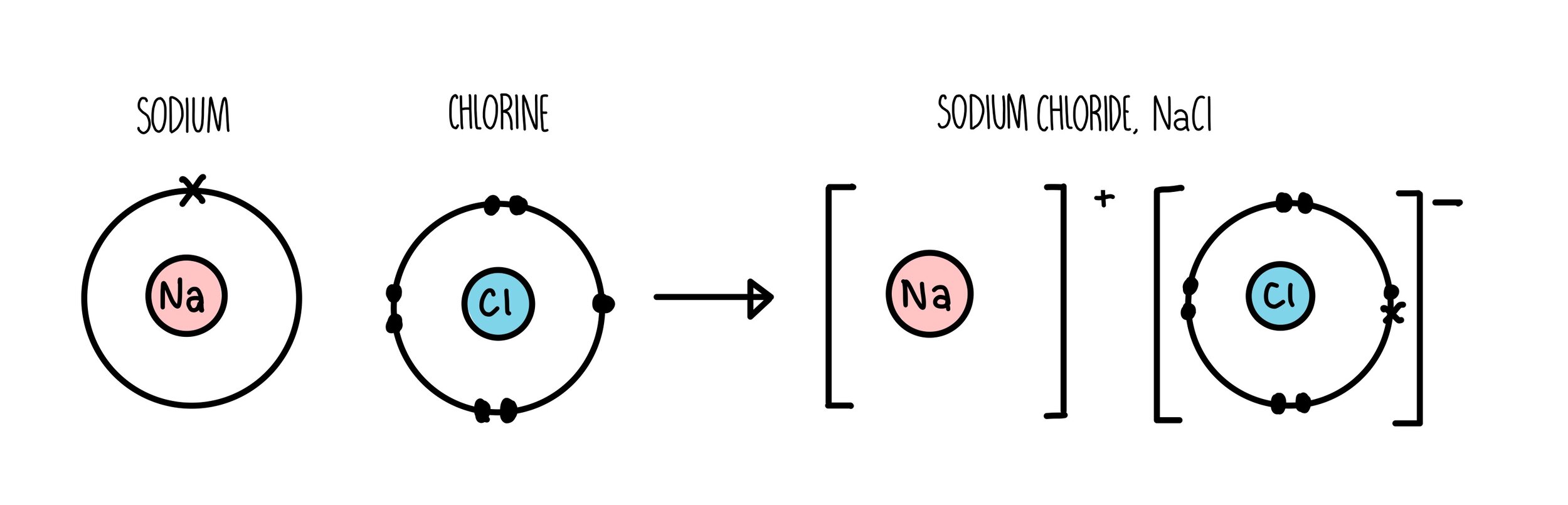
It’ll do almost anything it can to gain that electron. Chlorine, element 17, for example, has one too few electrons. (Image: Gravity Digital/Shutterstock) Formation of Ionic Bonding A ceramic mug is an example of ionic bonding. In such an event, the world of chemistry would be boring. Imagine what would happen if all combinations of electrons had exactly the same energy. , George Mason University In thinking about ionic bonding, the key is to remember that atoms or molecules with magic numbers of electrons are particularly stable, while atoms or molecules that are one or two electrons too many or too few are unstable.


 0 kommentar(er)
0 kommentar(er)
